Take-off with FUTURA
The digital compact solution for MDR-compliant documentation
With FUTURA fast and easy to the technical documentation of medical devices according to MDR (EU) 2017/745.

MDR: Simple, clear, fast and secure with FUTURA!
With FUTURA, the MDR becomes less burdensome, because we have compiled the documentation for your medical device already*!
* A large number of generic medical devices, especially surgical instruments, are already included in our FUTURA database solution.
As a manufacturer, you must generate, present and keep up-to-date all information about your medical device in accordance with the Medical Device Regulation. This involves an enormous amount of time, personnel and testing, and special expert knowledge is required.
The documentation is very costly and maintenance-intensive, as it shall always be up to date.
MDR Documentation means
- Big piles of documents
- Expert knowledge required
- High costs for laboratory testing
- Translations
- High research effort
FUTURA is Bundled Expert Knowledge
You can access the technical documentation for several thousand medical devices via our database. Your products are assigned to the systematic and generically structured documentation and continuously updated. This means your MDR-compliant documentation is always up to date and at your fingertips and downloadable for you 24/7/365.
The time, personnel and cost savings in your company are immense.
What does FUTURA do for your technical documentation?
- Process simplification
- Clarity
- Time saving
- Cost saving
- Reliability
We already did the job!
Through optimized digital processes and providing reprocessing test results for generic devices, MEDAGENT takes over 80% of the effort with FUTURA – with more benefits for you.
FUTURA offers you clarity and thus security for your technical documentation. From now on, submissions to Notified Bodies and Competent Authorities are always up-to-date and tangible!
Of course, you decide who gets to see which data – you have absolute control over your documentation and the transfer of data!
If you want to have a more specific product certified, you can of course also draw on our expert knowledge and range of services for MDR compliance.
- Systematic digital documentation
- Data master containing several thousand medical devices
- Less effort, more benefit - MEDAGENT takes over 80% of the job
- MDR-compliant technical documentation, always up-to-date
- Valid reprocessing evidence through worst-case testing
- Continuous expansion of existing validations
- Continuous updates
- New releases of product groups & risk classes
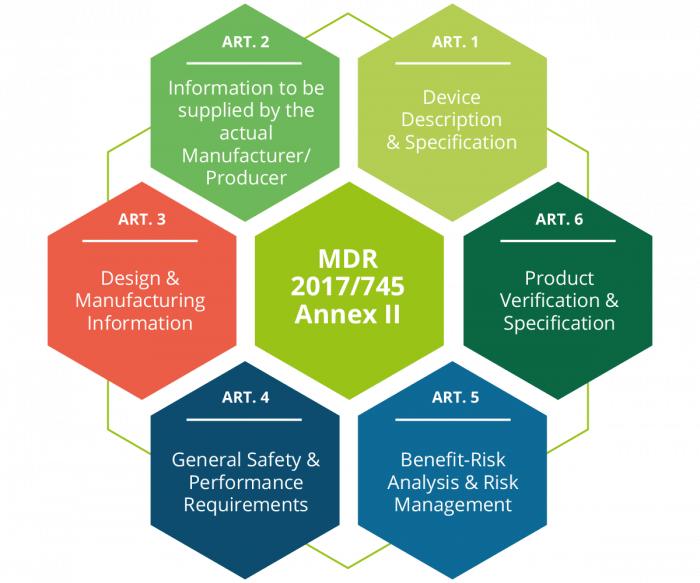
Digitalization of your documentation processes with FUTURA
Solutions for data management and information at the push of a button
MEDAGENT FUTURA and XCHANGE Service form the supporting pillars of a revolutionary merger in the MEDTEC industry. Securely and centrally, you can find all your data relating to your product. As a contract manufacturer, you store all the necessary information on your manufacturing processes here, and as a manufacturer, you can map your complete technical documentation via FUTURA. So you have everything about your product at a glance and available with just a few clicks.

- Reliable
- Centralized
- Trackable

- Completely
- Up-to-date
- Extendable
- Customisable
Once with FUTURA MDReady - always up to date!
Within FUTURA, we are constantly working on further developments. This means that not only does your documentation remain up to date, but standards are also constantly updated, new functional groups and further generic medical device groups are added, and official feedback and audit results from Notified Bodies and Competent Authorities are incorporated.
This makes revalidations, PMS reports and different languages child’s play to present and implement for you in your own company.
- Ongoing maintenance
- Incorporation of feedback from Notified Bodies and Competent Authorities
- Inclusion and updating of standards
- Continuous detailing and individualization of risk analyses
- Inclusion of further generic medical device groups (risk class I, Ir, Im, Is, IIa, IIb, III)
- Extension of existing reprocessing certificates
- Reprocessing evidence for other worst-case products
- Annual preparation of PMS reports with your data and FUTURA Benchmark
- Documents in different languages
- Licensing for approvals outside the EEA
- and much more!
Stay compliant with current norms and standards and free up your resources for innovation!
You want to start directly with FUTURA or have questions?
